
In nature, gold does not react with oxygen to form oxides like iron or copper. Whether buried in tombs, sand, or resting on the seabed for thousands of years, gold nuggets, grains, and bars continue to shine brightly.
So, if gold does not oxidize, what exactly is oxidized gold ore?
Understanding Oxidized Gold Ores
Oxidized gold ore refers to gold - bearing ore formed through natural oxidation processes, rather than gold oxides.
Such ores typically contain gold along with oxides of metals like iron, copper, and manganese. The main minerals are limonite and pyrite, with little or no sulfides, but they do include stable secondary minerals like gold - bearing iron hydroxides or hydrated iron oxides, along with some quartz.

Surface weathering transforms primary sulfide minerals into oxides and hydroxides, with gold potentially being embedded in hematite, goethite, or limonite.
Oxidized gold ore usually appears yellow to brown and has a loose texture. It is an important raw material for gold production.
How to Extract Gold from Oxidized Gold Ore?
In oxidized gold ore, most of the gold is contained within the main gangue minerals and the cracks of weathered metal oxides. The gold particle size varies greatly, and the mineral composition is relatively simple.
Before starting crushing and grinding processes, pre - treating the gold ore with a trommel screen is a crucial step for improving beneficiation efficiency, especially for oxidized gold ores containing clay.
Subsequently, gold extraction can be performed using methods such as gravity separation, cyanidation, or heap leaching.
Step 1: Ore Crushing
Crushing reduces the size of oxidized gold ore and is usually done using a dry process with two to three consecutive crushing stages: primary, secondary, and tertiary.

After crushing, a vibrating screen is used to classify the oxidized gold ore. Smaller particles pass through the screen openings while larger particles remain on the screen surface and are returned for further crushing.
Step 2: Ore Grinding
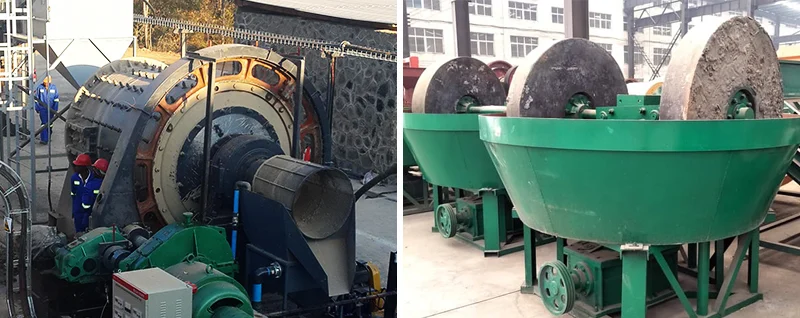
Grinding releases the gold from the gangue minerals in oxidized gold ore, creating favorable conditions for subsequent beneficiation. Common grinding equipment includes ball mills and wet pan mills.
Step 3: Ore Beneficiation
Oxidized gold ore beneficiation is a complex and critical process. Different extraction processes can be selected according to the nature of the ore and the form of gold. The following are several common beneficiation methods:
1. Gravity Separation
Gravity separation is a physical method that uses the difference in specific gravity between gold ore and gangue minerals for separation.
Commonly used oxidized gold ore gravity separation equipment includes shaking table, spiral chute, jig, etc.

2. Cyanidation
The cyanidation method is the most widely used chemical method for gold extraction. It is suitable for small and medium - scale mining of oxidized gold ores, especially those with fine particle size and high grade.
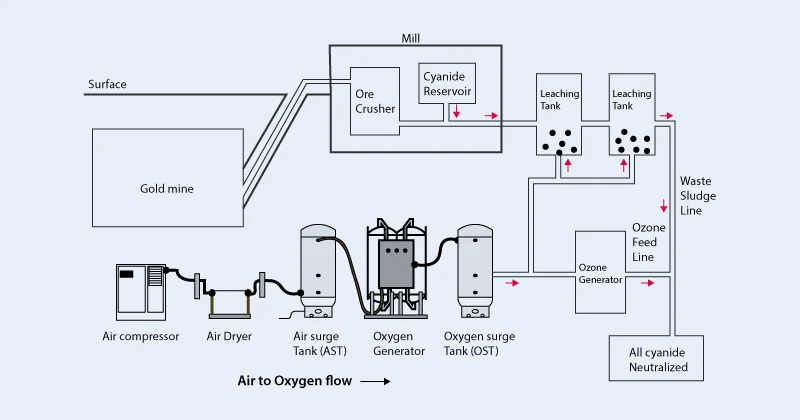
After grinding, the oxidized gold ore is mixed with cyanide solution, and the gold is dissolved in the liquid through leaching reaction, and then the gold is recovered by activated carbon adsorption or zinc precipitation.
Cyanidation offers high gold recovery rates and wide applicability but involves complex operations and high costs due to the need to manage cyanide waste.
3. Heap Leaching
Heap leaching is suitable for low - grade or large chunks of oxidized gold ore, often without prior grinding.
After crushing, the gold ore is stacked on a leak - proof heap leaching pad, forming an ore pile. Cyanide solution is sprayed over the pile to dissolve the gold, which is then recovered from the leach solution.
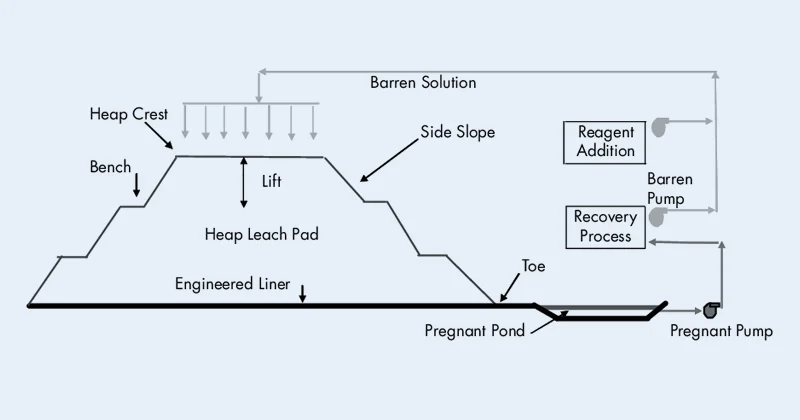
This process has low investment cost, simple operation, wide adaptability, and is suitable for large - scale extraction of low - grade gold ores. However, it requires specific ore particle size, as very fine particles may affect leaching efficiency.
These are the three main methods for processing oxidized gold ore. For partially oxidized ores, gravity separation and cyanidation are often combined. In fully oxidized ores, coarse gold is mostly recovered using gravity separation, while fine gold particles are treated with agitation cyanidation, and sand - sized particles are processed with percolation cyanidation.
Conclusion
The processing of oxidized gold ore depends on the ore's nature and the form of gold. By choosing the most suitable beneficiation methods and optimizing crushing, grinding, and separation processes, gold recovery rates can be maximized and economic benefits improved.
Shanxi United Chemical produces high - quality Sodium cyanide through advanced technology and equipment. For technical questions related to gold ore cyanidation, heap leaching, etc., you can consult us at any time. We help customers achieve more efficient and stable gold recovery.
- Random Content
- Hot content
- Hot review content
- Lead Chloride/ Lead (II) Chloride 98%
- Shock Tube Detonator
- Methanol Methyl alcohol 99.9% Industrial Grade Clear colorless liquid
- Cobalt Sulphate Heptahydrate
- Cupric Chloride 98%
- Diethylene Glycol
- Calcium Chloride 74% Flakes
- 1Discounted Sodium Cyanide (CAS: 143-33-9) for Mining - High Quality & Competitive Pricing
- 2China's New Regulations on Sodium Cyanide Exports and Guidance for International Buyers
- 3Sodium Cyanide 98% CAS 143-33-9 gold dressing agent Essential for Mining and Chemical Industries
- 4International Cyanide(Sodium cyanide) Management Code - Gold Mine Acceptance Standards
- 5China factory Sulfuric Acid 98%
- 6Anhydrous Oxalic acid 99.6% Industrial Grade
- 7Oxalic acid for mining 99.6%
- 1Sodium Cyanide 98% CAS 143-33-9 gold dressing agent Essential for Mining and Chemical Industries
- 2High Quality 99% Purity of Cyanuric chloride ISO 9001:2005 REACH Verified Producer
- 3Zinc chloride ZnCl2 for High Molecular Weight Polymers Initiator
- 4High Purity · Stable Performance · Higher Recovery — sodium cyanide for modern gold leaching
- 5High Quality Sodium Ferrocyanide / Sodium Hexacyanoferr
- 6Gold Ore Dressing Agent Safe Gold Extracting Agent Replace Sodium Cyanide
- 7Sodium Cyanide 98%+ CAS 143-33-9











Online message consultation
Add comment: