Rare Earth Mineral Processing Reagents: Collectors, Depressants, Frothers & Leaching Agents for Efficient and Sustainable Recovery
Rare earth elements (REEs) possess a range of exceptional physical and chemical properties, making them critical in various applications, from electronics to military uses. They are recognized as essential minerals by countries such as China, the United States, Japan, and Australia. However, rare earth minerals are abundant in variety but low in grade and are often closely associated with similar gangue minerals. Their beneficiation relies heavily on advancements in mineral processing reagents.
This article is geared towards the efficient beneficiation of rare earth resources. It summarizes the current state of research and development of flotation reagents for mineral-based rare earth ores, including collectors, depressants, activators, and frothers, along with their flotation mechanisms. The chemical beneficiation reagents for ion-type rare earth ores, including leaching agents and precipitating agents, are also discussed, covering their research status and leaching mechanisms. Furthermore, the current state of rare earth flotation collectors is evaluated, and future research directions for Rare earth mineral processing reagents are analyzed. This review aims to provide a reference for companies and professionals engaged in rare earth mineral processing and reagent development.
0 Introduction
Rare earth elements (REEs) include scandium, yttrium, and all 15 lanthanides, totaling 17 elements. These elements exhibit a range of exceptional physical and chemical properties, making them critical in various civilian and military sectors, including medical, energy, and defense industries. They are often referred to as "industrial vitamins," "miracle elements," "agricultural hormones," and "war metals," recognized as critical minerals by nations like the United States, China, Japan, Australia, Canada, and the European Union. According to the United States Geological Survey (USGS), as of 2022, the global rare earth oxide (REO) reserves stand at approximately 120 million tons, primarily concentrated in China (36.7%), Vietnam (18.3%), Brazil (17.5%), Russia (17.5%), India (5.8%), and Australia (3.3%).
The world's major rare earth mines include China's Bayan Obo, Maoniuping, and Ganzhou deposits, the Mountain Pass mine in the U.S., the Araxa and Minasu mines in Brazil, the Strange Lake deposit in Canada, the Mount Weld deposit in Australia, and the Zandkopsdrift deposit in South Africa. Additionally, China's southern provinces, including Jiangxi, Guangdong, Fujian, and Yunnan, are home to over 170 high-quality ion-adsorption rare earth deposits, serving as the global primary source of medium and heavy rare earth elements.
More than 250 types of rare earth minerals have been identified, with bastnäsite ((Ce, La)(CO3)F), monazite ((Ce, La)PO4), xenotime (YPO4), yttrialite (Y2FeBe(SiO4)2O2), and fergusonite (YNbO4) accounting for over 95% of the total mineral-based rare earth ores. However, these ores are often associated with quartz, fluorite, barite, feldspar, calcite, and other silicate gangue minerals, resulting in low-grade ores that are challenging to separate. As such, the beneficiation of rare earth ores often requires a combination of gravity separation, magnetic separation, and flotation to upgrade low-grade ores to industrial smelting-grade concentrates. In the case of ion-adsorption rare earth ores, rare earth elements are adsorbed as ions on mineral surfaces or within crystal layers, requiring chemical processing to extract rare earth oxides.
Whether dealing with mineral-based or ion-type rare earth ores, the application of beneficiation reagents is crucial in determining product grade, rare earth recovery rates, production efficiency, costs, and environmental impact.
This article focuses on the efficient beneficiation of rare earth resources, offering a detailed overview of the types, mechanisms, and research progress of flotation reagents (collectors, frothers, regulators) for mineral-based rare earth ores, as well as chemical beneficiation reagents (leaching agents, precipitating agents) for ion-type rare earth ores. It also presents future directions for research and development in rare earth mineral processing reagents, aiming to provide a reference for companies and researchers engaged in rare earth separation or industrial reagent development.
1 Rare Earth Flotation Collectors
Collectors play a crucial role in rare earth flotation by altering the surface hydrophobicity of target minerals, making them easier to attach to bubbles and improving their flotation properties. Based on functional groups, collectors for rare earth flotation can be classified into hydroxamic acids, fatty acids, phosphonic acids, and other reagents.1.1 Hydroxamic Acid Collectors
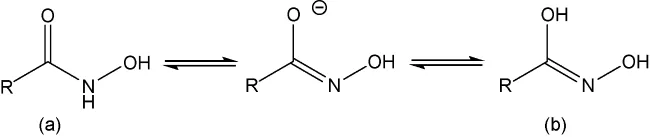
Hydroxamic acid collectors, developed in the 1980s, are the most commonly used reagents in rare earth flotation. Hydroxamic acids, also known as oximes, exist in two isomeric forms: oxime (keto structure) and hydroxamic acid (enol structure), with oxime being predominant. Both isomers dissociate to form identical anions during flotation.
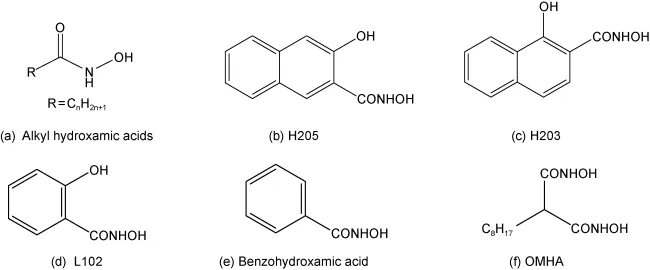
Common hydroxamic acid collectors used in rare earth flotation include C7-C9 alkyl hydroxamic acid, 2-hydroxy-3-naphthohydroxamic acid (H205), 1-hydroxy-2-naphthohydroxamic acid (H203), salicylic hydroxamic acid (L102), cycloalkyl hydroxamic acid, benzyloxamic acid, octyl malonic hydroxamic acid (OMHA), and other modified or mixed hydroxamic acid products, such as H316 (a modified H205), P8 (mainly hydroxynaphthohydroxamic acid), LF8# (98% hydroxynaphthohydroxamic acid), and collector 103 (salicylic hydroxamic acid). While hydroxamic acids show good selectivity for rare earth elements, they often require heating during flotation, leading to higher energy costs, and their synthesis can also be expensive.
1.2 Fatty Acid Collectors
Fatty acid collectors have been used in rare earth flotation since the 1950s when oleic acid was successfully applied to Mountain Pass in the United States. In China, systematic studies on the use of oleic acid and oxidized paraffin soap for rare earth flotation began in the 1960s.
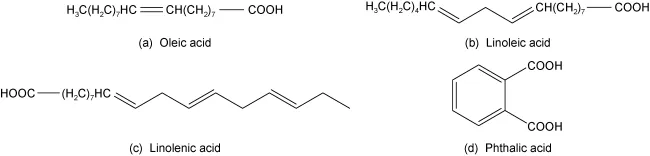
Fatty acid collectors are derived from natural vegetable or animal oils, typically composed of a mixture of C10-C20 saturated and unsaturated carboxylic acids or salts. Common reagents include oleic acid, sodium oleate, tall oil, oxidized paraffin soap, Bacchus fruit oil, phthalates, naphthenic acid, and oxidized petroleum derivatives. However, fatty acid collectors have lower selectivity for rare earth minerals and often require the addition of depressants and temperature adjustments to achieve effective separation.
The flotation of rare earth minerals using fatty acids is believed to involve a combination of physical adsorption, chemical adsorption, and surface chemical reactions.
1.3 Phosphonic Acid Collectors
Phosphonic acid (—P=O) and phosphonate (—O—P=O) collectors exhibit stronger flotation performance for metallic minerals compared to hydroxamic and fatty acid collectors. However, phosphonic acid collectors generally have lower selectivity.
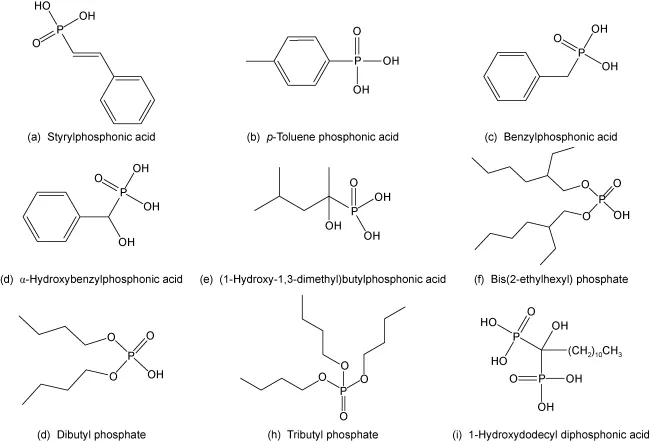
Currently used phosphonic acid collectors in rare earth flotation include styrene phosphonic acid, p-toluene phosphonic acid, benzyl phosphonic acid, α-hydroxybenzyl phosphonic acid, and commercial products such as P538 and Flotinor 1682.
1.4 Other Collectors
Other than hydroxamic acids, fatty acids, and phosphonic acids, a variety of novel collectors are being explored to improve rare earth flotation efficiency and selectivity. Some of these include sulfonates, thio-phosphates, and quaternary ammonium salts.
Sulfonates: Sulfonates have been reported to exhibit good selectivity and performance in flotation processes, but their application in rare earth mineral flotation is still in its early stages.
Thio-phosphates: These collectors are often used in sulfide mineral flotation, but research into their application in rare earth flotation is ongoing.
Quaternary Ammonium Salts: These compounds have been explored for their ability to float non-sulfide minerals, and some success has been reported in rare earth flotation. They operate through electrostatic attraction with negatively charged mineral surfaces.
Researchers are constantly experimenting with new reagents to enhance the effectiveness of rare earth mineral flotation, focusing on both improving recovery rates and reducing the environmental impact of these chemicals.
2 Depressants for Rare Earth Flotation
Depressants are essential in rare earth mineral flotation for selectively inhibiting gangue minerals, thereby improving the selectivity and yield of the target rare earth minerals. The primary gangue minerals associated with rare earth ores, such as quartz, calcite, and barite, often exhibit similar flotation behaviors, making their selective inhibition crucial.
Common depressants in rare earth flotation include water glass (sodium silicate), sodium fluoride, tannins, and starch.
2.1 Sodium Silicate (Water Glass)
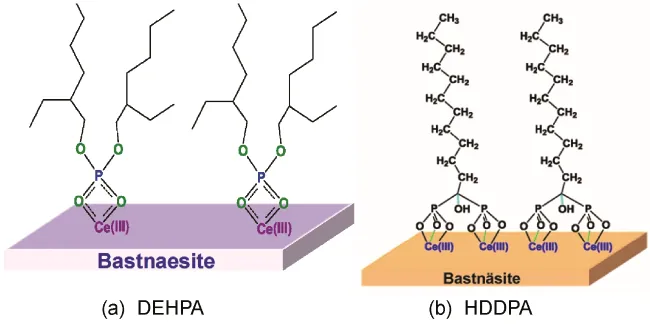
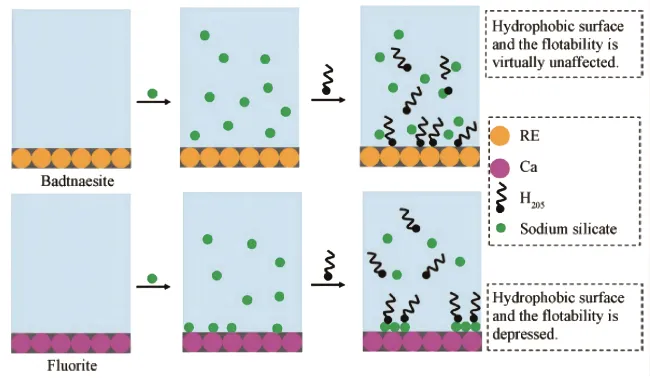
Sodium silicate, commonly known as water glass, is one of the most widely used depressants in rare earth flotation. It is used to inhibit silicate minerals such as quartz and feldspar. The mechanism of sodium silicate's depressive action is generally attributed to the formation of a silica layer on the surface of the gangue minerals, which prevents collector adsorption.
Water glass is an effective, low-cost depressant, but its performance can be influenced by factors such as pH, ion concentration, and reagent dosage. Researchers are exploring modified silicates and other chemical additives to improve the selectivity of water glass.
2.2 Sodium Fluoride
Sodium fluoride is used to depress calcite in rare earth flotation processes. Its depressive effect is based on the reaction between fluoride ions and calcium ions, forming an insoluble calcium fluoride film on the mineral surface, which prevents collector adsorption.
However, sodium fluoride is a highly toxic substance, and its use can pose environmental and safety concerns. As a result, researchers are actively seeking safer alternatives.
2.3 Tannins and Starch
Tannins and starch are examples of organic depressants used in rare earth flotation. Tannins, derived from plant materials, are used to depress gangue minerals such as barite and fluorite. Their mechanism involves complexation with metal ions on the mineral surface, reducing collector attachment.
Starch is commonly used as a depressant for hematite and other iron-bearing minerals in the flotation of rare earth minerals. The interaction between starch and minerals is typically physical, with the starch molecules adsorbing onto the mineral surface, preventing collector action.
2.4 New Depressants
The development of new depressants is an ongoing area of research in rare earth flotation. These novel reagents aim to improve the selectivity and reduce the environmental impact of the flotation process. Examples of recent developments include modified starches, synthetic polymers, and biodegradable organic depressants.
3 Frothers for Rare Earth Flotation
Frothers play a vital role in creating stable froth in flotation cells, enabling the separation of rare earth minerals from gangue materials. Frothers influence the bubble size, froth stability, and flotation kinetics. The most commonly used frothers in rare earth flotation are alcohol-based and ether-based reagents.
3.1 Alcohol-Based Frothers
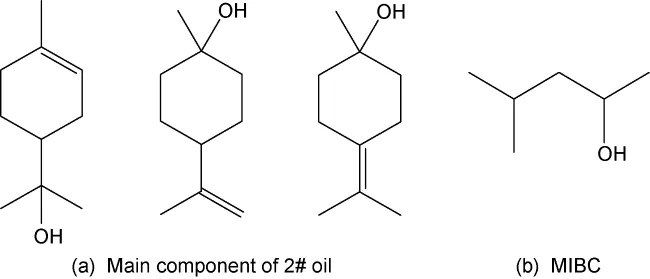
Alcohol-based frothers, such as methyl isobutyl carbinol (MIBC) and pine oil, are widely used in mineral flotation, including rare earth flotation. These frothers help to generate small, stable bubbles that enhance the flotation of fine particles.
Alcohol-based frothers are relatively inexpensive and effective, but their performance can vary depending on factors such as pH, mineral composition, and reagent interactions.
3.2 Ether-Based Frothers
Ether-based frothers, such as polypropylene glycol ethers (e.g., DF-250), are also commonly used in rare earth flotation. These frothers tend to produce finer bubbles and more stable froths compared to alcohol-based frothers. However, ether-based frothers can be more expensive and may require precise dosage control.
3.3 Novel Frothers
Research into new frothers for rare earth flotation focuses on improving selectivity and froth stability while minimizing the environmental impact. These include biodegradable frothers and frothers with improved resistance to the presence of oils and other contaminants in the flotation slurry.
4 Leaching Reagents for Ion-Adsorption Rare Earth Ores
Ion-adsorption rare earth ores are unique in that the rare earth elements are adsorbed on the surface of clay minerals rather than being locked in mineral structures. These ores are typically processed using leaching rather than flotation. Leaching agents play a critical role in this process by desorbing the rare earth ions from the clay surfaces.
4.1 Ammonium Sulfate Leaching
Ammonium sulfate is the most commonly used leaching agent for ion-adsorption rare earth ores. The ammonium ions in solution exchange with the rare earth ions on the surface of the clay minerals, releasing them into solution. This method is widely used because of its relatively low cost and simplicity.
However, ammonium sulfate leaching can cause significant environmental issues, particularly in terms of ammonium ion pollution. Efforts are being made to develop more environmentally friendly alternatives.
4.2 Sodium Chloride and Magnesium Sulfate Leaching
Sodium chloride and magnesium sulfate have been investigated as alternatives to ammonium sulfate. These reagents work through similar ion exchange mechanisms but have the advantage of being less harmful to the environment. However, they tend to be less effective in terms of recovery rates, and further research is needed to optimize their use.
4.3 Organic Leaching Agents
Organic leaching agents, such as citric acid and EDTA, are being explored as environmentally friendly alternatives to conventional inorganic leaching reagents. These organic compounds can effectively chelate rare earth ions, making them easier to extract from the ore. However, the cost of these reagents is a limiting factor for their widespread adoption.
5 Precipitating Agents for Ion-Adsorption Rare Earth Ores
Once rare earth ions are leached into solution, they need to be precipitated and recovered. Precipitating agents are used to form rare earth compounds that can be separated from the leach solution.
5.1 Ammonium Bicarbonate
Ammonium bicarbonate is commonly used to precipitate rare earth ions from leach solutions as rare earth carbonates. This reagent is effective and relatively low-cost, but it can produce large volumes of ammonium-containing wastewater, which poses environmental challenges.
5.2 Oxalic Acid
Oxalic acid is widely used to precipitate rare earth elements as rare earth oxalates, which can then be calcined to produce rare earth oxides. Oxalic acid is highly effective but can be more expensive than ammonium bicarbonate. Additionally, the handling of oxalic acid requires careful safety measures due to its toxicity.
5.3 New Precipitating Agents
Research is ongoing to develop more selective and environmentally benign precipitating agents for rare earth recovery. These include organic acids, biodegradable reagents, and ion-exchange resins.
6 Future Directions and Prospects
The future of rare earth mineral processing reagents lies in the development of more selective, efficient, and environmentally friendly reagents. Key areas for future research include:
Development of green reagents: The environmental impact of flotation and leaching reagents is a major concern, particularly in the context of rare earth processing. There is a growing need for the development of biodegradable, non-toxic reagents that can replace traditional chemicals like ammonium sulfate and oxalic acid.
Improvement in selectivity: New collectors, depressants, and frothers are needed to improve the selectivity of rare earth flotation, especially for low-grade and complex ores. This includes the exploration of new molecular structures and the modification of existing reagents.
Cost reduction: The high cost of some rare earth processing reagents, particularly hydroxamic acids and phosphonic acids, is a limiting factor for their widespread use. Future research should focus on the synthesis of more affordable alternatives or on improving the efficiency of existing reagents to reduce dosage requirements.
Environmental sustainability: With increasing regulations around the world aimed at reducing the environmental impact of mining operations, the development of environmentally sustainable rare earth processing technologies is becoming more important. This includes minimizing the use of harmful chemicals and reducing the generation of waste and pollution.
In conclusion, rare earth mineral processing is heavily dependent on the use of chemical reagents, and ongoing research is essential to improve the efficiency, selectivity, and sustainability of these reagents. The development of new, greener reagents will be critical for the future of rare earth beneficiation, as global demand for these critical minerals continues to rise.
- Random Content
- Hot content
- Hot review content
- Sulphuric Acid 98% Industrial Grade
- IPETC 95%Metal sulfide mineral collector Z-200
- Shock Tube Detonator
- Toluene
- Ammonium Persulfate Industrial Grade 98.5%
- Triethanolamine(TEA)
- lithium Carbonates 99.5% Battery Level or 99.2% Industry grade 99%
- 1Discounted Sodium Cyanide (CAS: 143-33-9) for Mining - High Quality & Competitive Pricing
- 2China's New Regulations on Sodium Cyanide Exports and Guidance for International Buyers
- 3Sodium Cyanide 98% CAS 143-33-9 gold dressing agent Essential for Mining and Chemical Industries
- 4International Cyanide(Sodium cyanide) Management Code - Gold Mine Acceptance Standards
- 5China factory Sulfuric Acid 98%
- 6Anhydrous Oxalic acid 99.6% Industrial Grade
- 7Oxalic acid for mining 99.6%
- 1Sodium Cyanide 98% CAS 143-33-9 gold dressing agent Essential for Mining and Chemical Industries
- 2High Quality 99% Purity of Cyanuric chloride ISO 9001:2005 REACH Verified Producer
- 3Zinc chloride ZnCl2 for High Molecular Weight Polymers Initiator
- 4High Purity · Stable Performance · Higher Recovery — sodium cyanide for modern gold leaching
- 5High Quality Sodium Ferrocyanide / Sodium Hexacyanoferr
- 6Gold Ore Dressing Agent Safe Gold Extracting Agent Replace Sodium Cyanide
- 7Sodium Cyanide 98%+ CAS 143-33-9











Online message consultation
Add comment: