Introduction
Research indicates that the treatment of copper and arsenic-containing waste acid employs a process that involves sulfide precipitation, followed by gypsum addition and electrochemical neutralization, ultimately leading to stable discharge that meets regulatory standards. In the sulfide precipitation step, a sodium sulfide solution with a mass concentration of 26% is automatically added to the copper and arsenic-containing waste acid under specific acidity conditions, resulting in the formation of CuS and As2S3 precipitates to remove copper and arsenic ions from the liquid phase. In actual production, sodium sulfide solution contains relatively high levels of insoluble impurities, and when exposed to air, it gradually oxidizes to form thiosulfate. Additionally, it has a high crystallization temperature, among other drawbacks. Therefore, finding a new sulfide agent to replace sodium sulfide would be of significant importance.

Solid Sodium Hydrosulfide
Process Principle
Constrained by the existing excess H2S absorption process, the treatment of copper and arsenic-containing waste acid using Sodium hydrosulfide is conducted based on the current equipment and process flow of the sulfuric acid workshop's waste acid and wastewater. This method employs a mixed solution of sodium hydrosulfide and sodium sulfide for the treatment.

Process Flow
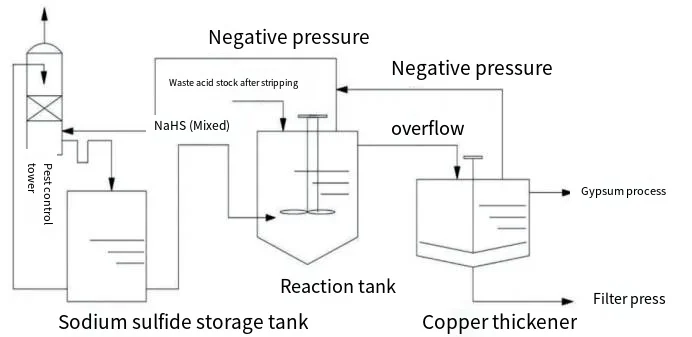
Figure 1 illustrates the process flow diagram for the treatment of copper and arsenic-containing waste acid using sodium hydrosulfide. The waste acid solution, after the removal of SO2 in the stripping tower, enters the sodium hydrosulfide reaction tank from the top. A mixed solution of sodium hydrosulfide and sodium sulfide is added from the bottom of the reaction tank. Under acidic conditions, H2S gas is generated and gradually rises, reacting with copper and arsenic from the top down to form CuS and As2S3 precipitates. These precipitates then overflow into the copper thickener for sedimentation. The supernatant is sent to the gypsum treatment process, while the bottom sludge is dewatered in a filter press before being sent for further processing. A small amount of unreacted H2S is drawn off by a negative pressure pipe to the detoxification tower, where it is absorbed by the circulating mixed solution of sodium hydrosulfide and sodium sulfide, ensuring that it meets discharge standards.
Experimental Method
A continuous production mode is adopted, with other processes and control parameters remaining unchanged. Sodium hydrosulfide reacts faster than sodium sulfide; if the concentration is relatively high, a large amount of hydrogen sulfide (H2S) is generated rapidly under acidic conditions. Apart from reacting with some of the copper and arsenic in the waste acid, the relatively excessive hydrogen sulfide will escape the liquid surface due to the agitation of the stirring paddle and be drawn off by negative pressure to the detoxification tower. The existing process uses sodium sulfide to absorb the excess hydrogen sulfide generated during the sulfide reaction (Na2S + H2S = 2NaHS). After trial use of sodium hydrosulfide, the sodium sulfide storage tank contains some sodium hydrosulfide, which reduces its ability to absorb excess hydrogen sulfide. To prevent incomplete absorption of hydrogen sulfide, which could lead to non-compliant emissions from the detoxification tower, water (condensate from the heating steam of the sodium sulfide storage tank) is added to the sodium sulfide storage tank during the trial period to maintain the concentration of the mixed solution of sodium sulfide and sodium hydrosulfide at approximately 15%.
Conclusion
After using a mixed solution of sodium hydrosulfide and sodium sulfide, the reagent cost for treating one cubic meter of waste acid is reduced to 52.5% of that of pure sodium sulfide, resulting in significant economic benefits.
The use of the mixed solution of sodium hydrosulfide and sodium sulfide has not affected the discharge indicators of the workshop; the wastewater discharge standards remain stable and compliant.
Due to the mixed use of sodium sulfide and sodium hydrosulfide, and with the existing detoxification tower process unchanged, there have been no instances of non-compliance in the emissions from the detoxification tower.
- Random Content
- Hot content
- Hot review content
- Booster(Detonating insensitive explosives)
- Sodium Peroxide
- Toluene
- Ammonium Persulfate Industrial Grade 98.5%
- 97% 2-Hydroxypropyl methacrylate
- Caprylic/capric triglyceride
- How does Sodium Ferrocyanide help in the mineral flotation process?
- 1Discounted Sodium Cyanide (CAS: 143-33-9) for Mining - High Quality & Competitive Pricing
- 2Sodium Cyanide 98% CAS 143-33-9 gold dressing agent Essential for Mining and Chemical Industries
- 3China's New Regulations on Sodium Cyanide Exports and Guidance for International Buyers
- 4International Cyanide(Sodium cyanide) Management Code - Gold Mine Acceptance Standards
- 5China factory Sulfuric Acid 98%
- 6Anhydrous Oxalic acid 99.6% Industrial Grade
- 7Soda Ash Dense / Light 99.2% Sodium Carbonate Washing Soda
- 1Sodium Cyanide 98% CAS 143-33-9 gold dressing agent Essential for Mining and Chemical Industries
- 2High Purity · Stable Performance · Higher Recovery — sodium cyanide for modern gold leaching
- 3Sodium Cyanide 98%+ CAS 143-33-9
- 4Sodium Hydroxide,Caustic Soda Flakes,Caustic Soda Pearls 96%-99%
- 5Nutritional Supplements Food Addictive Sarcosine 99% min
- 6Sodium Cyanide Import Regulations & Compliance – Ensuring Safe and Compliant Importation in Peru
- 7United Chemical's Research Team Demonstrates Authority Through Data-Driven Insights













Online message consultation
Add comment: